Electrochemical Storage: Lead- acid -Accumulator
Lead acid batteries
- The oldest type of rechargeable battery (1859)
- Low energy-to-weight, energy-to-volume ratios but ability to supply high surge currents (short burst of high power)
- Low cost
Components:
Positive and negative internal plates made of lead
Plate separators made of porous synthetic material
Electrolyte, a dilute solution of sulfuric acid and water (battery acid)
Plate separators made of porous synthetic material
Electrolyte, a dilute solution of sulfuric acid and water (battery acid)
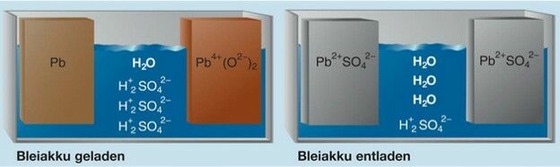
Basic reactions during charging and discharging
- Typical electrolysis: diluted sulfuric acid (water:acid ratio 3:1)
- Positive plate PbO2 (Lead dioxide)
- Negative plate Pb